🌏 Farm the Ocean
Give me half a tanker of iron and I’ll give you the next ice age.—John Martin, oceanographer
What if there was a way to capture more CO2 than we’ve ever dreamed, reduce the surface dedicated to farmland, plant more trees, increase life around the world, solve world hunger, grow the economy, and make money along the way?
In Seaflooding, I proposed turning deserts into seas teeming with life and bursting with economic opportunity.
What if we did the same thing, but opposite? What if we applied the idea to the biggest deserts on Earth:
As we saw in Why Are Tropical Waters Dead, and Can They Kill Europe?, massive swaths of our oceans have very little life. We call them ocean deserts:
Look at these three illuminating graphs.
On the left, you have the share of the Earth’s surface. You can see that the open ocean is about ⅔ of all the Earth’s surface. In the middle graph, you can see that, unfortunately, production of organic matter per m2 in the open ocean is as low as in extreme deserts. They are really like deserts!
Meanwhile, tropical rainforests account for about 3% of the Earth’s surface, but their production of organic matter per m2 is through the roof.
So the biggest surface is like a desert, while the most productive has a small area. The combination of these two means that open oceans and tropical rainforests are the two biggest producers of organic matter, accounting together for nearly half of all the Earth’s.
What if we had a region that was both big and productive? We could! Look at algal beds and reefs. They produce even more organic matter per m2 than tropical rainforests! Unfortunately, they only cover 0.1% of the Earth’s surface. If only we could expand them into the rest of the open ocean… we could turn them into the equivalent of sea forests.
Turning Deserts into Jungles
For most land deserts, we know exactly why they exist and what is needed to turn them into verdant forests: water.
Most deserts have plenty of sunlight. The soil is fertile. They just lack water. Release that constraint, and plants can combine the soil, the water, and the sun into organic matter.
Law of the Minimum
The Law of the Minimum states that there’s always only one factor that limits growth. Once this limiting factor is released, growth increases until it hits the next limiting factor. It’s a bit like filling a barrel with water.
The water level depends on the length of its staves. The shortest one will determine the amount of water. Increase that stave’s length, and the water will go up until it reaches the next stave1.
We see this everywhere in life. For example, Malthus’ hypothesis was that, historically, the main constraint on human population size was food. If you increased productivity, you didn’t make people richer, you just made more people. More food, more people. He feared we would run out of food, or that we would keep encroaching on productive land just to create more people.
This didn’t end up being the case, because it turns out once you release food as a constraint, people want other things, and these other things reduced natality around the world. The limiting factor (stave) of food was released, and other limiting factors emerged2.
But how did we release the food bottleneck? The Haber-Bosch Process, which allowed to transform energy into nitrogen fertilizer. Nitrogen is what plants needed the most but soils were lacking. It was the limiting factor on land. What’s the equivalent for the oceans? Plants there have all the water they need, so they’re not limited like deserts. Is nitrogen also the limiting factor ? If so, how can we change that? How can we introduce N at a massive scale in the ocean to create more life there?
Nitrogen in the Ocean
This is a map of nitrates3 in ocean surface water:
Compare this with the map of chlorophyll in the ocean:
Notice that in the regions where there are surface nitrates, there’s much more chlorophyll (ie, life). In the vast majority of the ocean, however, there isn’t much surface nitrogen. Is this why there’s not much life at all?
Look at this other map. It shows how much nitrogen there is compared to phosphorus.
Phosphorus us important because it’s also a typical nutrient missing for plants, as we’re going to cover in the premium article this week. You can see that in the regions where there’s oceanic life (North Pacific, Equatorial Pacific, Antarctic Ocean, North Atlantic), there’s much more nitrogen than phosphorus. And where there isn’t life, there’s broadly as much nitrogen as phosphorus. That tells us that the limiting factor is indeed nitrogen, not phosphorus4.
Tests have been carried out to fertilize seas with one, the other, or both. Fertilizing with both is always better, but in the ocean, nitrogen fertilization is much more important than phosphorus.
This explains why, when nitrogen makes it into the sea, life blossoms.
Algal Blooms
You might have heard of algal blooms in the context of toxic events that kill sea life.
This can happen through the following process:
A wave of nutrients enters water
Algae, phytoplankton, and equivalents consume them and reproduce
They keep doing it because algae and phytoplankton can reproduce very quickly.
So much so that, adhering to the Law of the Minimum, they reach the next limiting factor
Sometimes, that limiting factor is oxygen: they consume all of it by breathing
They also carpet the surface in green, preventing light from reaching below the surface and killing organisms that live from photosynthesis, like phytoplankton or algae
Fish can’t breathe and die
They rot and smell
Also, sometimes, cyanobacteria grows and produces toxic cyanotoxins
The wave of nutrients may also contain other compounds, like pesticides, herbicides, heavy metals, or something else, further polluting the waters
Given that this can happen, many people have a bad association with algal blooms. But that’s not any algal bloom, that’s a harmful algal bloom! Algal blooms can be caused by algae and phytoplankton that don’t reach the level of absorbing all oxygen from the water. Instead, they are simply a blooming of life: more phytoplankton, which can feed zooplankton and from there continue into the food chain to feed fish, dolphins, whales…
Nitrogen in the ocean is good the same way as water is good: they’re both necessary nutrients. An avalanche of nitrogen is not good for your health, the same way a flood of water isn’t either.
And algal blooms also happen in nature constantly. They happen at the mouth of the Amazon River, with all the nutrients its water leaches from the soil. They happen after every volcanic eruption that drops its mineral-rich ash in the ocean.
What we need is not to stop all types of nutrition to the ocean, for fear of creating harmful algal blooms of cyanobacteria. What we need is to understand the conditions under which adding nutrients to the ocean creates a thriving ecosystem, and those under which it kills everything.
The only way to do that is by trying. We could dump nitrogen into the sea, monitor how life grows in these areas, and adjust nutrients, their quantities, and locations to optimize life.
Here, we’re hitting a different problem though: producing all this nitrogen—which, as we saw, requires a lot of energy—to dump in the ocean would be expensive. Could we reduce the costs? Could we find a business model to finance the operations? Could it be a model that would also improve the environment in other ways?
Resurfacing Life
If only there was a way to bring existing nitrogen to the surface of the ocean without spending all this money to produce and then dump it in there…
Actually, there are nitrates in the ocean, just not where we need them to be. The maps above show nitrates at the surface level (top map) and 1000m below the surface (bottom map). There are plenty of nitrates in the depths! Why? They drop due to gravity and ocean downwelling, and are mainly from dead animals and fecal matter, from what I can gather.
This is also true for other nutrients, like iron5.
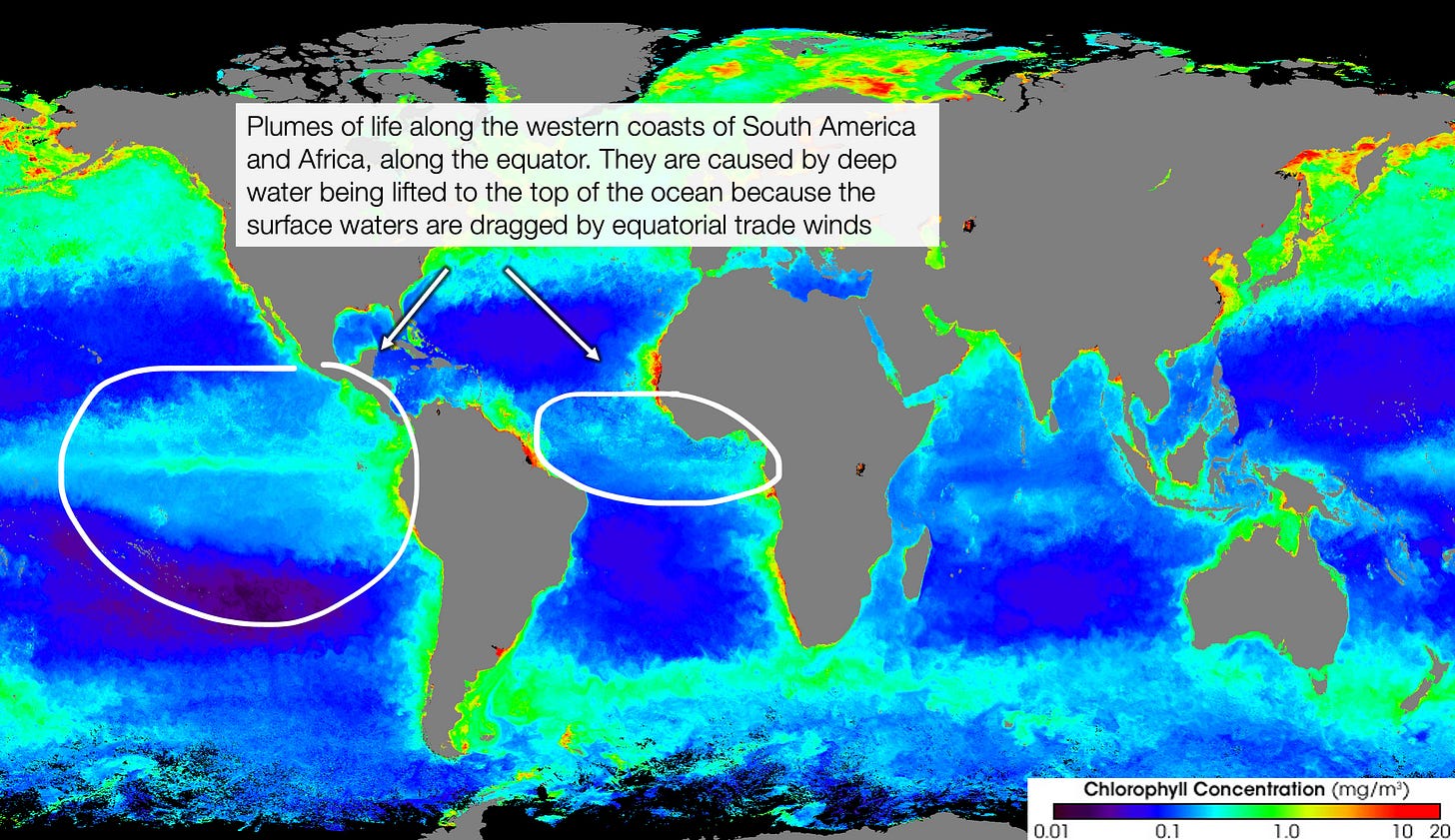
Which means that bringing these waters to the surface should increase nitrogen (and other nutrients)—and hence life. That’s indeed what happens, for example on the west coasts of South America and Africa, around the equator.
That’s why there are plumes of life near western equatorial coasts.
Could we use this concept and bring nutrients from the bottom to the surface of the ocean? How hard would it be?
There is a lot of research on this subject6.
This 2022 paper, for example, showed that you can bring deep ocean water to the surface, and when you do, phytoplankton explodes and permanently captures lots of CO2. This is unsurprising as it’s exactly the same as what happens naturally along western equatorial coasts.
Some papers study how to bring this water up using waves, others using offshore wind turbines and their existing shaft, others by pumping air. This study was able to use upwelling to replace toxic blooms with healthy ones. Meta-analyses are pretty enthusiastic about this topic.
But as far as I can tell, no companies are trying to use this to increase fish farm production. Why? Science takes decades to progress in this type of arena, because it sinks money. But a for-profit company could invest much more money in much less time. Why is nobody doing it?
Pumping Iron
In fact, we might not even need to pump deep water up to the surface. Let’s look again at the map of chlorophyll vs surface nitrates:
In some areas, there’s much more chlorophyll than the nitrates would suggest: equatorial Atlantic, north Atlantic, and mid-Indian ocean. This indicates that you don’t need massive amounts of nitrates—or other nutrients—to create more life. Something else might be limiting growth in these areas.
This is most obvious when you look at the opposite: regions that have lots of nitrates (and generally also other nutrients) but weirdly don’t have the corresponding amount of life. The three main examples are the north Pacific, equatorial Pacific, and the Antarctic Ocean. The biggest example is the Antarctic (Southern) Ocean:
Here’s another view:
Nitrogen is not the limiting factor there. And neither are H, O (water), C (CO2 from the air), or P:
So what is limiting phytoplankton growth here? Iron.
Iron is crucial. Blood is red because it contains the iron ion Fe3+. It’s necessary for photosynthesis and respiration. It can also help acquire other key nutrients, like phosphorus, and more importantly, nitrogen.
So the idea would be to drop iron in the ocean, which would create phytoplankton blooms, which would feed zooplankton, then krill, fish, dolphins, whales…
All these animals are made of a lot of carbon, extracted from the ocean and air by the phytoplankton. So the more iron we drop in, the more carbon we extract. Around 10% of these animals and their feces fall to the bottom of the ocean as sediments and remain there7.
If we do it in a broadly confined environment in the ocean, we could harvest the fish and use it to replace other meats. It would be much cheaper and healthier to drop iron in the ocean and harvest the fish than to have millions of square kilometers of pasture for animals like cows8. This would allow us then to convert these pastures back to forest, capturing even more CO2 and improving land ecosystems.
It would also allow us to counter a terrible trend, where oceans have been losing about 1% of their phytoplankton every year since 1950, for a total decrease of 40%!
The good thing is that iron is very plentiful on land—up to 11% of the dust in the west Australian desert! You can just take that and dump it in the ocean—although it would benefit from enrichment9.
This wouldn’t single-handedly solve climate change, but it could be a big contributor to CO2 capture10, harnessing up to 3 gigatons of CO2 per year (about 8% of all annual CO2 emissions) with just ~200k tons/year of iron, for a ratio of 5000x CO2 to iron. Other calculations suggest 1000x-100,000x ratios of carbon captured to iron dropped11. The only limit of this type of intervention would be hitting other limiting nutrients12.
But the point of trying these things is to understand how they work so we can effectively adjust them. It’s in nobody’s interest to create a dead zone!
Would It Work?
This is not some crazy scheme. It’s just replicating what the Sahara does naturally, by dropping its sands on both the Amazon Rainforest and the Atlantic, fertilizing both.
It’s also what happens naturally after volcanic eruptions: there’s often an algal bloom afterwards. And we’ve been doing it inadvertently by burning fossil fuels and wood. For example, soot from the 2019-2020 Australian wildfires caused a bloom between New Zealand and South America that may have removed as much as 150 to 300 million tons of carbon.
If we look back in history, over the last million years, every time there was a peak in iron in ocean sediments, there was also a peak in photosynthetic organisms, measured by the amount of opal13.
We also see that times with low carbon in the atmosphere correspond to times with lower temperatures, but also more dust!
So have we tried to replicate this voluntarily? Would it work? Yes, we have, more than 14 times so far! These tests show that it works: it does create algal blooms.
For example, here you can see satellite data showing a chlorophyll explosion after a polemic iron fertilization experiment14. First, we can see the chlorophyll the month before the dump and after:
And here is a comparison of August 2008 (a good year for plankton) vs 2012.
So these experiments clearly work15.
A study from just five months ago showed that iron fertilization could double fish farming profits!
Are We Doing It Then?
We’re NOT!
I’ve looked into this and it’s hard to find companies doing mariculture (ocean farms). Most water farming is done with freshwater:
There are a handful of mariculture operations in the Western World, but as far as I can tell, none are attempting iron fertilization, and definitely none of them has any grand scheme for doing it at a large scale16. Why is that?
Regressive environmentalists.
Here’s the logic: We’ve done a lot of research about iron fertilization, and it’s very promising. But there are plenty of things we don’t know yet. We don’t know how much carbon could be captured, so we don’t know the upside of this. But we know the downsides: it could create fish kills; the droppings of the additional animals and fertilizer could pollute the bottom of the ocean; it could create imbalances in the ecosystem; if we farm fish that are not local, we contain them, and they escape, they might invade local ecosystems17; concentrated farm fish could have disease outbreaks that they pass on to the free populations… It’s a complicated solution that requires lots of infrastructure and could divert attention from other solutions we favor.
For example:
It is difficult if not impossible to detect and describe important effects that we know might occur months or years later. Some possible effects, such as deep-water oxygen depletion and alteration of distant food webs, should rule out ocean manipulation. History is full of examples of ecological manipulations that backfired.—John Cullen, oceanographer at Dalhousie University.
Although iron addition stimulates phytoplankton growth, it remains unclear whether it significantly increases the transfer of carbon from atmosphere to the deep ocean.—NIWA18
This type of approach meant that, in 2008, the United Nations Convention on Biological Diversity put in place a moratorium on all ocean-fertilization projects apart from small ones in coastal waters. Five years later, the London Convention on ocean pollution adopted rules for evaluating such studies.
Conclusion
The result now is that everybody is blocked from experimenting with this because there’s “not enough data”. This common issue paralyzes regressive environmentalists: the upside is known, but the downside is imagined. And imagination is always wilder than reality, so we end up doing nothing. Instead, this is the perfect example of something we should be testing. And not through science, which has made great progress on this topic, but that has taken 50 years! It’s too slow.
Based on everything we know, this is a no-brainer:
Easy to do by dumping dust in the ocean
Create lots of life in the process
Farm or catch a lot of fish
Make lots of money for fish farms, reversing a trend of impoverishment of many fishing-based economies
Fish protein could replace much less environmentally-friendly meat. This would allow us to reclaim pasture (and farmland for animal feed) and convert it back into forest.
Lots of CO2 would be captured and sunk to the bottom of the ocean
And fish farmers shouldn’t do it for the CO2, because it’s hard to prove right now. They should do it for the fishing money. And once they understand better how it works, they could get remunerated for the carbon they sink too.
We should let the private sector explore this, with some guidelines and red lines rather than forbidding the activity altogether. In fact, governments should be investing in this!
The future of fishing is not catching wild fish anymore. It’s engineered:
While researching this article, I stumbled upon a company that is doing something even cleverer than iron fertilization. I might invest in them. I’ll write about it in the premium article.
Let’s continue this trend. Let’s keep pushing humanity forward instead of being paralyzed by fear.
In this week’s premium article, I’ll also write about why nitrogen is so important, what other elements are important, and how that explains how fertilizers are the way they are today. For example, why did I talk about nitrogen, phosphorus, and iron today? There’s a crucial reason that explains the origin of life.
What did you think about this week’s article? Let me know by leaving your comments below!
The company I mention that’s doing something cleverer than iron fertilization could also be a candidate to win the Xprize $100M challenge. If you know a company that wants to operate in the area, please let me know. I’d love to talk with them. I might even want to invest in the right one.
I got interested in this topic by reading Nick Szabo’s fantastic Elements, evolution, and the nitrogen crisis. His entire blog is a gold mine.
It’s a bit like the critical path in project management, the bottleneck in production, or the theory of constraints.
Nitrogen in a common form that organisms can consume, NO3.
Spelling out the logic: organic matter needs 16x more N than P. So what you should expect is that in places where there is less N than that (compared to P), the limiting factor would be N, and life couldn’t breed as successfully. This is what we see, with regions where N and P are close to equal being completely dead. Meanwhile, areas that get closer to the 16:1 ratio have more life. When these regions have even more than 16:1, there’s even more life. This wouldn’t be the case if P had become a limiting factor. So we can conclude P is usually common enough in these waters to not be the limiting factor.
This makes sense, as sealife near the surface consumes the nutrients from there, where they have access to both light and air.
And this is just a sample.
This is how fossil oil and natural gas were formed, so this would literally be sending the oil that we’ve been consuming back down.
By the way, apparently the iron limitation is also valid on land.
Plenty of soils have 3-8% of iron in them, which is enough. The west Australian desert dust contains 11% iron. But dumping that soil might not be optimal, as the iron might just sink, and other nutrients from other types of soils (such as silicates) might be beneficial. So we might want to include some additives: for example, rice husk ash, which contains 90% - 98% silica for proper growth of diatoms that sink rapidly and help sequester CO2. Others could have other functions, such as wood sawdust for iron to bind to and float on for months. More details here.
The worst of which would be oxygen, which could cause a fish kill and a dead zone. But phytoplankton—and also algae like kelp—could use the iron to increase photosynthesis, which would add oxygen to the sea. This just needs to be done thoughtfully, so that it doesn’t benefit toxic cyanobacteria more than the healthier types of photosynthetic organisms.
A material often used in jewelry, which marine researchers use as an indicator of phytoplankton abundance. Opal is secreted by diatoms as material to form diatom shells. Diatoms are among the most common and important kinds of phytoplankton. The idea is that they ate iron, reproduced, and their shells fell to the bottom of the ocean, forming the opal over the last million years.
In this case, it was an entrepreneurial person helping locals with their annual salmon harvest. Their harvests had been dwindling for a long time, and they wanted to do something about it. This created controversy, as it was done without the required permits.
A couple of papers looked into the impact of this action. This paper suggests the evidence supports that zooplankton did grow there. So did this paper:
At its location in the central GoA, this experiment induced the largest phytoplankton bloom observed over the past 10 years, even stronger than that caused by iron addition associated with the Kasatochi volcano in 2008. Due to its limited spatial and temporal scales, in terms of broad impacts, however, estimated total annual carbon drawdown by this experiment is an order of magnitude smaller than that of the Kasatochi volcano and annually recurring Haida eddies.
China has published a lot of papers on the topic, and South Korea and Japan also have many papers. I wouldn’t be surprised if they led the way.
Forget that this is open ocean… This does happen when it’s not open ocean, and hence why this has been used as an argument.
Here’s another NIWA quote:
The carbon regulatory market requires further standards for carbon sinks, which include verification of carbon storage, and permanence, by which carbon must be retained for 100 years or greater. It is easier to follow the transfer and storage of carbon in a terrestrial system than in the more dynamic ocean. Not only will the carbon fixed by phytoplankton sink 1000s of metres through the water column, but it is also transported laterally over 1000s of kilometres by ocean currents. Determining the permanence of carbon sequestration in the ocean is therefore highly challenging, expensive and currently not considered viable with existing technology. Yet this is essential, particularly as the 100-year time horizon, which is generally considered to be depths greater than 500 m, is only reached in certain regions, with sub-surface water returning to the surface within decades in other regions.
Don’t get me wrong, the facts are accurate! But this should not prevent us from iron dumping for fishing. And we should not avoid solutions just because we can’t measure them, if everything we know suggests they’re great solutions.




























Excellent summary on helping ocean deserts bloom and sequester carbon. Phytoplankton feeds the food chain When those animals respire below 100m (e.g. anchovies at night) that CO2 dissolves and stays in solution for decades, even 100s of years. When organic matter from decaying zooplankton and diatoms remineralize into their base elements, that carbon sequesters for generations. When iron is added and phytoplankton grows, the blooms often attract fish and whales that cause vertical mixing. This brings nitrogen and nutrients from deeper levels to the surface which can keep the bloom going. Mimicking nature by adding iron and missing minerals is an inexpensive way to sequester carbon and help restore our oceans. Kevin Wolf, Co-chair Ocean Iron Fertilizatioin Alliance, https://oifalliance.org kevinjwolf @ gmail.com
Another fascinating article!. Thanks!
Living in the Sonoran desert of Tucson, here is the biggest problem. While obviously we need water and more of it, we lack an essential ingredient - Humidity.
Even fully watered plants will desiccate by mid day in our 95 to 105F, 5 to 15% RH. And we have dust.